Alumni
Mohammad Alhudaithi
Bio
Mohammad is a 10th grade student from Riyadh, Saudi Arabia. He has a deep interest in chemistry, especially organic chemistry and computational chemistry. He hopes to one day employ computers to aid in organic chemistry research. In his free time he enjoys video games, coffee, and speedcubing.
Project: Chemical Bonds: Molecular Orbitals for One-Electron Diatomic Molecules
Goal
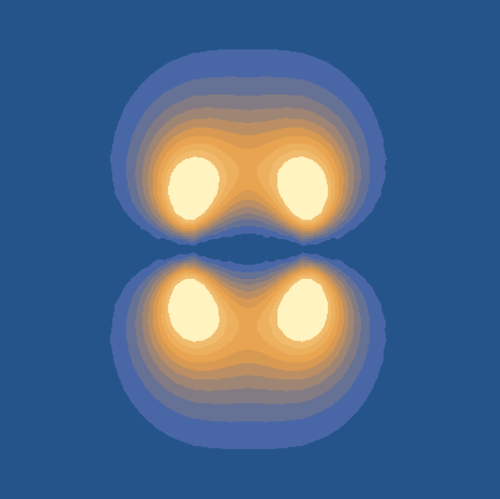
Molecular orbital (MO) theory is among the fundamental models describing molecular structures. Chemical reactivity highly depends on two features of MO, the probability distribution and corresponding energy level. The goal of this project is to calculate these features for different combination of atomic orbitals of one-electron diatomic molecules as a function of internuclear distance. Using the approximation for constructive and destructive linear combination of atomic orbitals, we numerically calculated the relevant integrals for Hamiltonian, overlap, Coulomb and resonance integrals. With the values of a 2x2 Hamiltonian, we found the eigenvalues, which are energy levels. Finally, we manipulated the intermolecular distance to illustrate how energy levels depend on the mentioned parameters.
Summary of Results
We implemented the wave function for hydrogen atoms in spherical and Cartesian coordinates. Using the aforementioned wave functions, we graphed the S, Pz, Px and Py orbitals. The latter two were calculated using the linear combination of the complex solutions for ml=-1,1. We graphed hybridized atomic orbitals and overlay them to observe the 3D geometry of an atom of that electronic structure. The next step was calculating the energy of molecular orbitals. This was done by solving the overlap, Coulomb and resonance integrals and using the numbers to calculate the energy of the bonding and antibonding orbitals for a given distance. This made it possible to produce a distance/energy graph. It is also possible to animate the changing shape of a molecular orbital as the distance between to atoms decreases.
Future Work
The clearest path forward is to graph more complex atoms and molecules; doing so would require more complicated wave functions and much more processing power. Another possibility is graphing different combinations of atomic orbitals. Another intriguing prospect is predicting the 3D shape of organometallic complexes.